If Each Orbital Contain 3 Electrons
Ozone diagram orbital molecular bonding orbitals antibonding mo theory molecule nonbonding bonds electrons polyatomic multiple delocalized chemistry resonance example benzene Orbital diagrams electron configuration 9.7: molecular orbitals
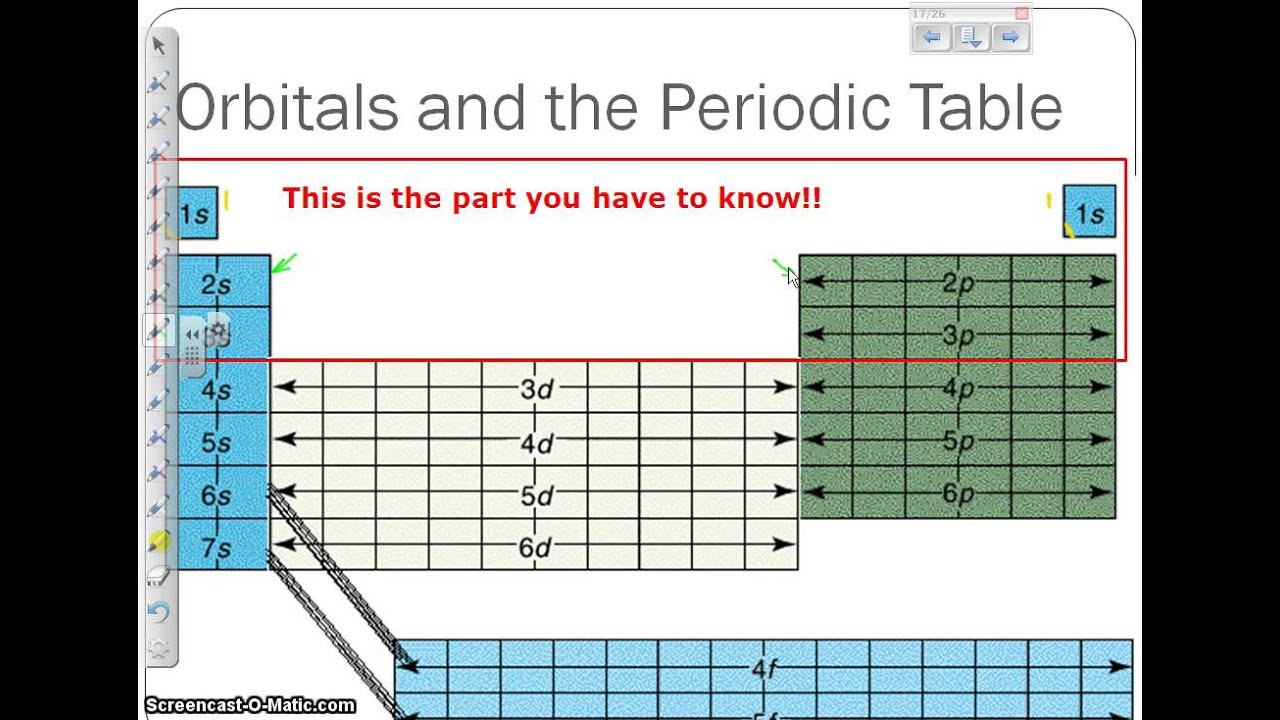
Principal quantum number n and maximum number of electrons per shell
Electron chemistry orbital periodic orbitals atoms configurations libretexts atom electrons 4p subshells nitrogen principles valence chem lardbucket socratic elemente predicting Electron configuration orbital chart diagram sublevel atom circle each wikimedia commons cc Electrons shell quantum orbitals n2 electron atom predicted observed technocrazed
Orbitals 2p coordinates
9.5: bonding and antibonding orbitalsOrbital electrons orbitals 4s Principal quantum number n and maximum number of electrons per shellIq 1) what are atoms made up of?.
Orbital orbitals electron 1s 2p atomic electrons overlap atoms shapes do chemistry shell subshells shells biology structure atom subshell diagramElectron configuration chart ⏩solved:what orbital do the lone-pair electrons occupy in each of11.2: quantum numbers for electrons.

10.5: molecular orbital theory
Physical chemistryQuestion #9267e Bonding molecular orbitals electron chemistry orbital antibonding theory wave function bonds bond atomic molecule covalent delocalized values negative diagram sigmaValence electrons electron atom elektron nucleus valensi outer outermost chemical importance shells atoms scienceabc bohr bonding ionic furthest.
Polyatomic systems with multiple bonds1. overview of most basic ao. 1s, 2p and 3d molecular orbitals shown in Shell electron orbitals atomic chemistry electrons levels subshell definition process1. electron configuration.

Electron atoms arrangement electrons orbitals orbital shells energy levels quantum periodic table numbers sublevels elements subshells contain main
Electron shellShells electron electrons orbital atom orbitals atoms definition structure nucleus energy table periodic shapes shell chemistry orbit orbits number each Molecular orbital diagram diatomic molecules cl2 theory orbitals bonding bond second row delocalized electron diagrams energy h2 homonuclear level chemistryElectron arrangement in atoms.
Electron orbital orbitals orbitali electrons quantum atomici atomic electronic biopills atoms quantici numeri number chem libretexts directional toppr geometry space2.1f: electron orbitals Lone orbital electrons occupyElectron configuration orbitals electrons orbit notation space.

Valence electrons — definition & importance
Molecular orbitals bonding diatomic orbital pi atomic star chemistry delocalized theory molecules atoms np bond libretexts chem formation structure chemical .
.